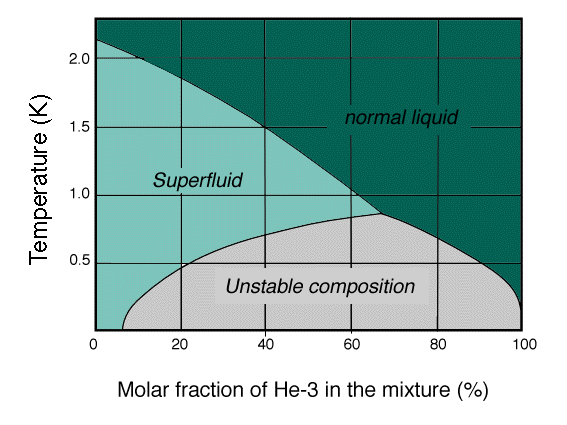
The principle of dilution refrigeration was suggested by F. London around 1950 ,when it became evident that a liquid mixture of two helium isotopes 3He and 4He would undergo a phase transition at temperatures below 1 K.

The phase diagram of a liquid helium mixture at low temperatures shows that dilution cooling can take place only below the highest temperature of unstable composition, the tricritical point. The mixture can either be a normal liquid, a fermi liquid , a superfluid or a stratified combination depending on the average concentration and the temperature .
In absolute values the cooling power is small but as the cooling takes place at very low temperatures the amount of energy to be removed upon cooling is also small. The success of the refrigerator is very much dependent upon the precooling of the incoming 3He by heat exchangers.
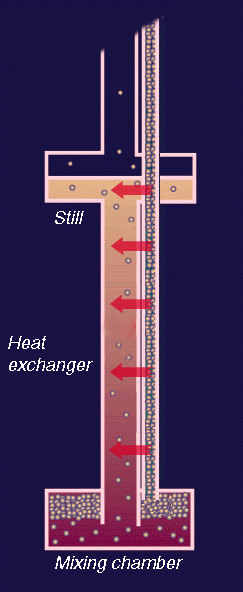
Schematic flow diagram of a dilution refrigerator
3-APR-95